Page 5 - Al-Rashed Newsletter February 2021
P. 5
POTPOURRI
COVID VACCINE
THE VACCINE TESTING PROCESS
Researchers are currently testing 71 vaccines in clinical trials on humans, and 20 have reached the final
stages of testing. At least 78 preclinical vaccines are under active investigation in animals. Leading
vaccines are Pfizer BioNTech, Moderna, Gamaleya, Oxford Astrazenaca etc
PRECLINICAL TESTING: Scientists test a new vaccine on cells and then give it to animals such as mice
or monkeys to see if it produces an immune response.
PHASE 1 SAFETY TRIALS: Scientists give the vaccine to a small number of people to test safety and
dosage, as well as to confirm that it stimulates the immune system.
PHASE 2 EXPANDED TRIALS: Scientists give the vaccine to hundreds of people split into groups, such
as children and the elderly, to see if the vaccine acts differently in them. These trials further test the
vaccine’s safety.
PHASE 3 EFFICACY TRIALS: Scientists give the vaccine to thousands of people and wait to see how
many become infected, compared with volunteers who received a placebo. These trials can determine if
the vaccine protects against the coronavirus, measuring what’s known as the efficacy rate. Phase 3 trials
are also large enough to reveal evidence of relatively rare side effects.
EARLY OR LIMITED APPROVAL: Many countries have given emergency authorization based on
preliminary evidence that they are safe and effective. China, Russia and other countries have begun
administering vaccines before detailed Phase 3 trial data has been made public. Experts have warned of
serious risks from jumping ahead of these results.
APPROVAL: Regulators review the complete trial results and plans for a vaccine’s manufacturing, and
decide whether to give it full approval.
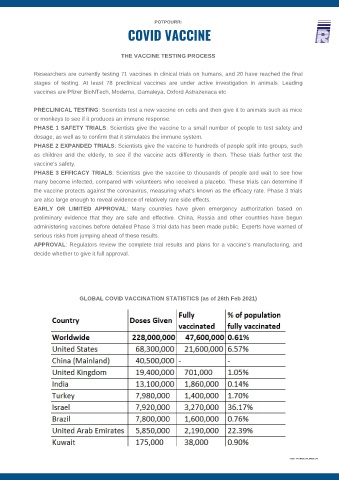
GLOBAL COVID VACCINATION STATISTICS (as of 26th Feb 2021)
MR. GOLDY CHADHA
KEY ACCOUNT MANAGER
Source- NYTIMES.com, Github.com